Viktor Reinhardt and Matt Rossell
Animal Welfare Institute
Washington, DC
Injurious self-biting is one of the most serious problems in primate colonies (Niemeyer, Gray, & Stephen, 1996). "Approximately 10% of captive, individually-housed monkeys engage in the disturbing phenomenon of self-injurious behavior (SIB). To date, no adequate explanation or effective therapy has been developed for this disorder" (Jorgensen et al., 1996; cf. Novak et al., 1998). In rhesus macaques - the predominant species found in laboratories - the incidence of self-biting may be as high as 14% (recorded in a colony of 188 single-caged males; Jorgensen, Kinsey, & Novak, 1998). Individuals affected with this "behavioral pathology" (Erwin, & Deni, 1979, p. 4) repeatedly bite parts of their own bodies (Figure 1) while intermittently showing signs of intense excitation such as threatening, trembling, head jerking and piloerection (Reinhardt, 1999; cf. Tinklepaugh, 1928).

CAUSE
There is no discernible influence of rearing history on the development of self-biting (Bayne, Haines, Dexter, Woodman, & Evans, 1995). While self-biting is rather common in primates reared in isolation (Sackett, 1986), it is by no means restricted to animals with impoverished early experience (Erwin, Mitchell, & Maple, 1973; Bayne et al., 1995).
The behavior pattern typically occurs in emotionally disturbing situations, over which the subject has no control (Tinklepaugh, 1928; Erwin, Mitchell, & Maple, 1973; Gluck, & Sackett, 1974; Anderson, & Chamove, 1981). Separation of affectionate companions (Redican & Mitchell, 1973; Chamove, Anderson, & Nash, 1984), separation of sexual partners (Maple, Erwin, & Mitchell, 1973), excessive disruption of daily routines such as cage transfers (Jorgensen, Kinsey, & Novak, 1998), presence of fear-inducing personnel (Berkson, 1968; Fittinghoff et al., 1974; Allyn, Deyme, & Begue, 1976; Pond, & Rush, 1983; Reinhardt, 1999), or simply being confined alone in a cage can be such distressing experiences that they prompt self-biting behavior. The National Research Council (1998) warns that "prolonged individual housing is probably an influential contributing factor" of "severe self-directed biting" (p. 34) and, therefore, recommends that every effort should be made to house the animals socially rather than individually. This is in line with a fundamental rule of good animal husbandry: "Animals should be housed with the goal of maximizing species-specific behaviors and minimizing stress-induced behavior. For social species, this normally requires housing in compatible pairs or groups" (National Research Council, 1996, p. 22).
EFFECT
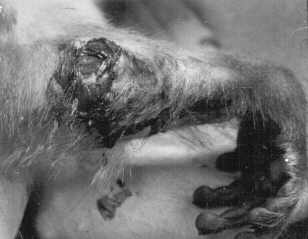
Self-biting often goes unnoticed because subjects most of the time do not break the skin while biting themselves (Reinhardt, & Reinhardt, 2001). In a case study of seven rhesus macaques, self-biting was associated with no visible wound in 43% (3/7) of cases, with abrasion(s) in 28.5% (2/7) of cases and lacerations in 28.5% (2/7) of cases (Reinhardt, 1999). Lacerations often require veterinary attention, and "research has shown that approximately 10% of captive, individually housed monkeys have had some veterinary record of self-injurious behavior in their life-time" (Jorgensen, Kinsey, & Novak, 1998, p. 187; cf. Platt, Kinsey, Jorgensen, & Novak, 1996). Self-biting may occur with sufficient vigor to break bones (Sackett, 1986), and the self-inflicted wound(s) can be so severe that surgery is needed to save the limb (Figure 2).
TREATMENT
Distracting three individually housed rhesus macaques with a food delivering apparatus reduced the incidence of motor stereotypies but not of self-biting behavior (Preilowski, Reger, & Engele, 1988). In another study with two rhesus macaques, self-biting declined in frequency, while motor stereotypies increased in the presence of a foraging apparatus (Watson, 1992). Obviously, "the ability of environmental enrichment devices to ameliorate ... self-aggressive behavior in laboratory primates is unclear" (Watson, Cosby, & Lee-Parritz, 1993, p. 356).
In a more representative investigation with nine single-caged rhesus macaques, it was found that access to puzzle feeders or grooming boards had no effect on self-injurious biting even though all subjects used the gadgets as intended (Kinsey et al., 1996; Kinsey, Jorgensen, & Novak, 1997). In fact, some monkeys got so excited while extracting peanuts from the puzzle that they actually bit themselves (Novak et al., 1998). These findings lead to the conclusion that self-injurious biting "cannot be remediated through simple environmental changes alone" (Kinsey, Jorgensen, & Novak, 1997, p. 123).
The twice daily oral administration of guanfacine - an alpha2 receptor agonist - halted self-biting in three monkeys. Discontinuation of treatment, however, resulted in a reoccurrence of this behavior pathology (Macy et al., 1999). Clinical assessment suggested that guanfacine decreased the high level of emotional arousal that commonly accompanies self-biting.
In a behavioral assessment of adult female long-tailed macaques "self-abusive behaviors [self-biting, self-hitting, hair-pulling] were recorded in five out of ten subjects when singly housed, but were completely absent after pair formation" (Line et al., 1990a, p. 4). The therapeutic effect of a social companion is also confirmed in rhesus macaques. Qualitative observations of juveniles suggested that self-biting was noticeably reduced in animals who were transferred from single- to pair-housing conditions (Bushong, Schapiro, & Bloomsmith, 1992). These findings were confirmed in a quantitative study of seven adults (three females, four males; Reinhardt, 1999). Transfer from single housing to compatible same-sex pair-housing arrangements had a therapeutic effect on pathological self-biting behavior in each of the seven subjects. "The conspicuous excitation and self-biting in the presence of personnel was abandoned immediately on the day of pair formation in three animals or gradually within the next two months in the four others. The behavior pattern was no longer witnessed thereafter" over a follow-up period of one year or longer (Reinhardt, 1999, p. 4). In two additional cases, one adult female and one adult male rhesus were cured from self-biting by permanently pairing each of them with a naturally weaned infant (Reinhardt et al., 1997; Figure 3).

DISCUSSION
Self-biting is the most serious behavioral pathology in captive macaques. The strikingly high level of emotional excitation along with the self-inflicted physical trauma suggest that the self-biting subject is reacting to a distressing situation. Since self-biting typically occurs in individually caged - rather than group-housed - subjects, the social deprivation associated with singe-caged housing is probably the primary, constantly active factor for the manifestation of this behavioral problem. Being confined in a single-cage without option to meet one of the basic requirements for well-being, namely companionship, is an extremely disturbing situation for nonhuman primates. It has been demonstrated in macaques that individual caging may constitute such a potent stressor as to produce immunosuppression (Lilly, Mehlman, & Higley, 1999; cf., Line et al, 1993; Schapiro et al, 2000), increase the susceptibility to diarrhea (Schapiro, & Bushong, 1994) and promote the development of coronary atherosclerosis (Shively, Clarkson, & Kaplan, 1989). It should be remembered that self-injurious behavior is also shown in human primates who are kept in solitary cells, and "these `cages' are so terrible that many prisoners prefer to maim themselves rather than stay there" (Yaroshevsky, 1975; p. 445). Regardless of these intrinsic problems "single or individual caging systems are the basic or staple housing used for primates" (Rosenberg, & Kesel, 1994, p. 459), and macaques in particular (National Research Council, 1998) in research laboratories.
In the U.S. there are an estimated 15,000 individually caged macaques. If 10% of these animals exhibit visible injuries resulting from self-biting, and another 10% show unnoticed self-biting behavior, than about 3,000 animals (20%) are affected by this gross behavioral pathology. It stands to reason to assume that these 3,000 individuals have no scientific value since research data collected from them are confounded by the subjects' high emotional disturbance, and hence cannot be considered scientifically valid (cf. Russell, & Burch, 1959; Novak, & Bayne, 1991; American Medical Association, 1992; Fuchs, 1997; Woolley, 1997). The secondary pathophysiological effect of self-biting may also interfere with any scientific investigation.
The seriousness of self-biting is highlighted by the fact, that this behavioral syndrome unlike other disorders is resistant to occupational therapy attempts (National Research Council, 1998). A foraging device, a grooming board or a toy will not cure a monkey of self-biting. Even though medical treatment is not a cure, it may at least halt self-biting thus providing time to correct extraneous stressors that trigger this behavior. The principal stressor is obviously the absence of a companion and in primates, like in most other social animals, the presence of a compatible social partner acts as a powerful buffer against extraneous stress (Bovard, 1959; Mason, 1960; Epley, 1974; Coe, Franklin, Smith, & Levine, 1982; Coelho, Carey, & Shade, 1991; Gust, Gordon, Brodie, & McClure, 1994). It is, therefore, not surprising that the transfer from solitary confinement to pair-housing arrangements is a reliable cure of self-biting behavior in macaques, and probably also in other primate species. The social companion meets a constant need and, therefore, provides a lasting cure. Other environmental enrichment options may distract an animal sufficiently to inhibit self-biting. But this effect will always come to an end once the subject has satisfied his or her appetite in food treats delivered by the foraging device, or has lost interest in the grooming board or in the toy.
There are a number of practical and safe options of transferring single-caged long-tailed macaques (Lynch, 1998), pig-tailed macaques (Byrum, & St. Claire, 1998), stump-tailed macaques (Reinhardt, 1994a), and rhesus macaques (Reinhardt, 1994b) - including social isolates (Reinhardt, 1990) - to compatible pair-housing arrangements without affecting the animals' stress status and health (Reinhardt, Cowley, & Eisele, 1991; Reinhardt, & Hurwitz, 1993; Schapiro, Bloomsmith, Kessel, & Shively, 1993; Eaton, Kelley, Axthelm, Iliff-Sizemore, & Shiigi, 1994; Schapiro, & Bushong, 1994) and without interfering with husbandry practices and common research protocols (Reinhardt, & Dodsworth, 1989; Reinhardt, Houser, & Eisele, 1989; Reinhardt, & Reinhardt, 2001). These options will have to be implemented on a much more consistent basis not only to "address the social needs of nonhuman primates" in accordance with current federal law (United States Department of Agriculture, 1991, p. 6499) but also to deal with the ethically and scientifically unacceptable problems of self-biting behavior.
The transfer of a self-biting subject to compatible group-housing arrangements is similarly effective as the transfer to pair-housing (senior author's unpublished observations; cf. Missakian, 1972; Bayne, Dexter, & Suomi, 1991; Hartner et al., 2000). However, under the constraints of the research laboratory it may be difficult to provide the necessary management conditions that will make the group-integration successful. Moreover, it also has to be assured that living in a group will, indeed, be less stressful for the subject than living alone (cf. Bernstein, Gordon, & Rose, 1974; Goo, & Sassenrath, 1980; Line, Morgan, Roberts, & Markowitz, 1990b; Reinhardt, Reinhardt, & Houser, 1986; Rolland, 1991; Schapiro, & Bushong, 1994; Bürge, Panoussis, & Weber, 1997).
The frequent occurrence of self-biting in individually caged macaques is an alarming sign that the housing conditions are not appropriate. In order to prevent the development and reduce or eliminate the occurrence of self-biting in caged macaques the animals' inherent social needs must be addressed with much greater consistency. No exemptions should be granted - except for veterinary health-care reasons - to the rearing and housing of primates in species-appropriate, compatible social settings. Serious efforts must be made to remove the avoidable pain and distress resulting from pathological self-biting, in the interests of both animal based science as well as animal welfare.
REFERENCES
Allyn, G., Deyme, A., & Begue, I. (1976). Self-fighting syndrome in macaques: 1. A representative case study. Primates, 17, 1-22.
American Medical Association (1992). Use of Animals in Biomedical Research - The Challenge and Response - An American Medical Association White Paper. Chicago: AMA. Group on Science and Technology.
Anderson, J. R., & Chamove, A. S. (1981). Self-aggressive behaviour in monkeys. Current Psychological Reviews, 1, 139-158.
Bürge, T., Panoussis, B., & Weber, H. (1997). Primate housing facilities for pharmaceutical research in Switzerland (an example). Primate Report, 49, 19-22.
Bayne, K., Dexter, S. L., & Suomi, S. J. (1991). Social housing ameliorates behavioral pathology in Cebus apella. Laboratory Primate Newsletter, 30 (2), 9-12.
Bayne, K., Haines, M., Dexter, S., Woodman, D., & Evans, C.(1995). Nonhuman primate wounding prevalence: A retrospective analysis. Lab Animal, 24 (4), 40-44.
Berkson, G. (1968). Development of abnormal stereotyped behaviors. Developmental Psychology, 1, 118-132.
Bernstein, I. S., Gordon, T. P., & Rose, R. M. (1974). Factors influencing the expression of aggression during introductions to rhesus monkey groups. In R. L. Holloway (ed), Primate Aggression, Territoriality, and Xenophobia (pp. 211-240) New York: Academic Press.
Bovard, E. W. (1959). The effects of social stimuli on response to stress. The Psychological Review, 66, 267-277.
Bushong, D., Schapiro, S. J., & Bloomsmith, M. A. (1992). Self-aggression in nonhuman primates: A review of its development/possible causes, methods of therapeutic treatment, and its relevance to the zoo situation. American Zoo and Aquarium Association (AZA) Regional Conference Proceedings, 723-728.
Byrum, R., & St. Claire, M. (1998). Pairing female Macaca nemestrina. Laboratory Primate Newsletter, 37 (4), 1.
Chamove, A. S., Anderson, J. R., & Nash, V. J. (1984). Social and environmental influences on self-aggression in monkeys. Primates, 25, 319-325.
Coe, C. L., Franklin, D., Smith, E. R., & Levine, S. (1982). Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology and Behavior, 29, 1051-1057.
Coelho, A. M., Carey, K. D., & Shade, R. E. (1991). Assessing the effects of social environment on blood pressure and heart rates of baboon. American Journal of Primatology, 23, 257-267.
Eaton, G. G. et al. (1994). Psychological well-being in paired adult female rhesus (Macaca mulatta). American Journal of Primatology, 33, 89-99.
Epley, S. W. (1974). Reduction of the behavioral effects of aversive stimulation by the presence of companions. Psychological Bulletin, 81, 271-283.
Erwin, J., Mitchell, G., & Maple, T. (1973). Abnormal behavior in non-isolate-reared rhesus monkeys. Psychological Reports, 33, 515-523.
Erwin, J. , & Deni, R. (1979). Strangers in a strange land: Abnormal behavior or abnormal environments? In J. Erwin, T. Maple, & G. Mitchell (eds), Captivity and Behavior (pp. 1-28) New York: Van Nostrand Reinhold.
Fittinghoff, N. A., Lindburg, D. G., Gomber, J., & Mitchell, G. (1974). Consistency and variability in the behavior of mature, isolation-reared, male rhesus macaques. Primates, 15, 111-139.
Fuchs, E. (1997). Requirements of biomedical research in terms of housing and husbandry: Neuroscience. Primate Report, 49, 43-46.
Gluck, J., & Sackett, G. (1974). Frustration and self-aggression in social isolate rhesus monkeys. Journal of Abnormal Psychology, 83, 331-334.
Goo, G. P., & Sassenrath, E. N. (1980). Persistent adrenocortical activation in female rhesus monkeys after new breeding group formation. Journal of Medical Primatology, 9, 325-334.
Gust, D. A., Gordon, T. P., Brodie, A. R., & McClure, H. M. (1994). Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology and Behavior, 55, 681-684.
Hartner, M. K., Hall, J., Penderghest, J., White, E., Watson, S., & Clark, L. (2000). A novel approach to group-housing male cynomolgus macaques in a pharmaceutical environment. Contemporary Topics in Laboratory Animal Science, 39 (4), 67.
Jorgensen, M. J., Novak, M. A., Kinsey, J., Tiefenbacher, S., & Meyer, J. S. (1996). Correlates of self-injurious behavior in monkeys. XVIth Congress of the International Primatological Society/XIXth Conference of the American Society of Primatologists, Abstract No. 767.
Jorgensen, M. J., Kinsey, J. H., & Novak, M. A. (1998). Risk factors for self-injurious behavior in captive rhesus monkeys (Macaca mulatta). American Journal of Primatology, 45, 187.
Kinsey, J. H., Jorgensen, M. J., Platt, D. M., Hazen, T. J. (1996). Food puzzle feeders: Effects on self-biting and stereotypy in individually housed monkeys. XVIth Congress of the International Primatological Society/XIXth Conference of the American Society of Primatologists, Abstract No. 683.
Kinsey, J. H., Jorgensen, M. J., & Novak, M. A. (1997). The effects of grooming boards on abnormal behavior in rhesus monkeys (Macaca mulatta). American Journal of Primatology, 42, 122-123.
Lilly, A. A., Mehlman, P. T., & Higley, J. (1999). Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. American Journal of Primatology, 48, 197-223.
Line, S. W., Morgan, K. N., Markowitz, H., Roberts, J., & Riddell, M. (1990a). Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Laboratory Primate Newsletter, 29 (4), 1-5.
Line, S. W., Morgan, K. N., Roberts, J. A., & Markowitz, H. (1990b). Preliminary comments on resocialization of aged macaques. Laboratory Primate Newsletter, 29 (1), 8-12.
Line, S. W., Shively, C. A., Heise, E. R., Rabin, B. S., & Cohen, S. (1993). Influence of single caging on cellular immune function in female cynomolgus macaques (Macaca fascicularis). American Journal of Primatology, 31, 328.
Lynch, R. (1998). Successful pair-housing of male macaques (Macaca fascicularis). Laboratory Primate Newsletter, 37 (1), 4-5.
Macy, J. D., Beattie, T. A., Morgenstern, S. E., & Arnstern, A. F. T. (1999). The use of guanfacine to control self-injurious behavior in nonhuman primates. Abstracts of the AALAS Meeting, 9.
Mason, W. A. (1960). Socially mediated reduction in emotional responses of young rhesus monkeys. Journal of Abnormal and Social Psychology, 60, 100-110.
Missakian, E. A. (1972). Effects of adult social experience on patterns of reproductive activity of socially deprived male rhesus monkeys (Macaca mulatta). Journal of Personality and Social Psychology, 21 (1), 131-134.
National Research Council (1996). Guide for the Care and Use of Laboratory Animals, 7th Edition. Washington: National Academy Press .
National Research Council (1998). The Psychological Well-Being of Nonhuman Primates. Washington: National Academy Press.
Niemeyer, C., Gray, E. G., & Stephen, T. (1996). Improving the psychological well-being of nonhuman primates by providing appropriate therapeutic devices. XVIth Congress of the International Primatological Society/XIXth Conference of the American Society of Primatologists, Abstract No. 678.
Novak, M. A., & Bayne, K. (1991). Monkey behavior and laboratory issues. Laboratory Animal Science, 41 (4), 306-307.
Novak, M. A., Kinsey, J. H., Jorgensen, M. J., & Hazen, T. J. (1998). Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. American Journal of Primatology, 46, 213-227.
Platt, D. M., Kinsey, J. H., Jorgenson, M. J., & Novak, M. A. (1996). Factors affecting the expression of self-injurious behavior in rhesus monkeys (Macaca mulatta). XVIth Congress of the International Primatological Society/XIXth Conference of the American Society of Primatologists, Abstract No. 768.
Pond, C., & Rush, H. G. (1983). Self-aggression in macaques: five case studies. Primates, 24, 127-134.
Preilowski, B., Reger, M., & Engele, H. (1988). Combining scientific experimentation with conventional housing: A pilot study with rhesus monkeys. American Journal of Primatology, 14, 223-234.
Redican, W. K., & Mitchell, G. (1973). The social behaviour of adult male-infant pairs of rhesus macaques in a laboratory environment. American Journal of Physical Anthropology, 38, 523-526.
Reinhardt, V. (1990). Social enrichment for laboratory primates: A critical review. Laboratory Primate Newsletter, 29 (3), 7-11.
Reinhardt, V. (1994a). Social enrichment for previously single-caged stumptail macaques. Animal Technology, 5, 37-41.
Reinhardt, V. (1994b). Pair-housing rather than single-housing for laboratory rhesus macaques. Journal of Medical Primatology, 23, 426-431.
Reinhardt, V. (1999). Pair-housing overcomes self-biting behavior in macaques. Laboratory Primate Newsletter, 38 (1), 4.
Reinhardt, V., Reinhardt, A., & Houser, W. D. (1986). Hair pulling-and-eating in captive rhesus monkeys. Folia Primatologica, 47, 158-164.
Reinhardt, V., Houser, W. D., Eisele, S., & Champoux, M. (1987). Social enrichment with infants of the environment for singly caged adult rhesus monkeys. Zoo Biology, 6, 365-371.
Reinhardt, V., & Dodsworth, R. (1989). Facilitated Socialization of Previously Single Caged Adult Rhesus Macaques (Videotape with accompanying text). Madison: Wisconsin Regional Primate Research Center.
Reinhardt, V., Houser, W. D., & Eisele, S. (1989). Pairing previously singly caged rhesus monkeys does not interfere with common research protocols. Laboratory Animal Science, 39, 73-74.
Reinhardt, V., Cowley, D., & Eisele, S. (1991). Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. Journal of Experimental Animal Science, 34, 73-76.
Reinhardt, V., & Hurwitz, S. (1993). Evaluation of social enrichment for aged rhesus macaques. Animal Technology, 44, 53-57.
Reinhardt, V., & Reinhardt, A. (2001). Environmental Enrichment for Caged Rhesus Macaques (Macaca mulatta) - Photographic documentation and literature review (Second Edition). Washington: Animal Welfare Institute.
Rolland, R. M. (1991). A prescription for psychological well-being. In M. A. Novak, &A. J. Petto (eds), Through the Looking Glass. Issues of Psychological Well-being in Captive Nonhuman Primates (pp. 129-134) Washington: American Psychological Association.
Rosenberg, D. P., & Kesel, M. L. (1994). Old-World monkeys. In B. E. Rollin, &M. L. Kesel (eds), The Experimental Animal in Biomedical Research. Volume II, Care, Husbandry, and Well-Being - An Overview by Species (pp. 457-483) Boca Raton: CPR Press.
Russell, W. M. S., & Burch, R. L. (1959). The Principles of Humane Experimental Techniques. London: Methuen & Co.
Sackett, G. P. (1986). Abnormal behavior in laboratory-reared rhesus monkeys. In M. W. Fox (ed), Abnormal Behavior of Animals (pp. 293-331) Philadelphia: Saunders.
Schapiro, S. J., Bloomsmith, M. A., Kessel, A. L., & Shively, C. A. (1993). Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science, 37, 251-263.
Schapiro, S. J., & Bushong, D. (1994). Effects of enrichment on veterinary treatment of laboratory rhesus macaques (Macaca mulatta). Animal Welfare, 3, 25-36.
Schapiro, S. J., Nehete, P. N., Perlman, J. E., & Sastry, K. J. (2000). A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Applied Animal Behaviour Science, 68, 67-84.
Shively, C. A., Clarkson, T. B., & Kaplan, J. R. (1989). Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis, 77, 69-76.
Tinklepaugh, O. L. (1928). The self-mutilation of a male Macacus rhesus monkey. Journal of Mammalogy, 9, 293-300.
United States Department of Agriculture. (1991). Title 9, CFR (Code of Federal Register), Part 3. Animal Welfare; Standards; Final Rule. Federal Register, 56, 6426-6505.
Watson, L. (1992). Effect of an enrichment device on stereotypic and self-aggressive behaviors in singly-caged macaques: A pilot study. Laboratory Primate Newsletter, 31(3), 8-10.
Watson, L., Cosby, R., & Lee-Parritz, D. E. (1993). Behavioral effects of enrichment devices on laboratory primates with stereotypic and self-directed behavior. American Journal of Primatology, 31, 355-356.
Woolley, A. P. A. H. (1997). Requirements of biomedical research in terms of housing and husbandry for non-human primates: Pharmacology & Toxicology. Primate Report, 49, 37-41.
Yaroshevsky, F. (1975). Self-mutilation in Soviet prisons. Canadian Psychiatric Association Journal, 20, 443-446.
Reproduced with permission of Lawrence Erlbaum Associates
from Journal of Applied Animal Welfare Science 4(4), 285-294, 2001