Experiments on animals (“in vivo” experiments) have long been the norm for learning about human health and disease, because testing on live animals enables researchers to investigate how chemicals, drugs, and disease affect a whole body, including the complex interactions between organs, tissues, and other biological systems. However, there are both ethical and scientific concerns about animal experimentation. Research shows that as many as 90 percent of preclinical studies—studies that explore underlying mechanisms of disease in animals and can cause extensive suffering—ultimately fail to translate into successful treatments, diagnostics, or disease prevention for humans.

Historically, experimental technologies that do not rely on the use of animals have been simplistic, isolated, and two-dimensional (e.g., growing cells in petri dishes and examining them under a microscope), which limits their applicability to humans in the real world. But recent technological advancements have generated much more complex and human-relevant methodologies, significantly improving the feasibility of replacing animals in experimentation. This article offers an overview of these innovative “new approach methodologies” (NAMs), as well as an assessment of how close we are to their widespread adoption.
New approach methodologies
The term “new approach methodologies” typically refers to research and testing techniques that don’t involve the use of live animals. This term is often used in the scientific community instead of “nonanimal methods” to signify that the primary focus of NAMs is to acquire more accurate, human-relevant data, regardless of whether the technologies provide direct replacements for existing experimental methods involving animals. NAMs can be divided into three broad categories: in chemico, in vitro, and in silico.
In chemico experiments, the simplest NAMs, do not require live organisms or even entire cells. Instead, biological molecules (e.g., proteins, DNA) are mixed into a test solution to explore what chemical reactions will occur. For example, rather than testing products for potential eye damage by applying chemicals directly to the eye of an awake, restrained rabbit (the infamous Draize test), scientists can add ocular proteins to a test solution containing the chemical and measure protein breakdown as a proxy for ocular irritation.
In vitro refers to experimentation on cells outside a living organism. While there is a long history of coaxing simple cell cultures to flourish in petri dishes and test tubes, scientists have more recently developed ways to grow cells in dynamic, three-dimensional microenvironments, including “organoids” and “organs-on-a-chip.” Organoids are microscopic 3D versions of organs grown from stem cells, which are able to divide, differentiate, and self-organize into the entire array of cell types in a given organ. Organs-on-a-chip (aka “organ chips”) are small plastic devices that contain tiny hollow channels lined with living human cells. Within organ chips, researchers can apply biomechanical pressures that catalyze the transfer of nutrients, waste, and drug molecules across channels, mimicking how organs function in live bodies (e.g., molecules moving from the bloodstream into the air channels of a breathing lung). Virtually any organ can be mimicked using this technology, and multiple different organ chips can be linked together.
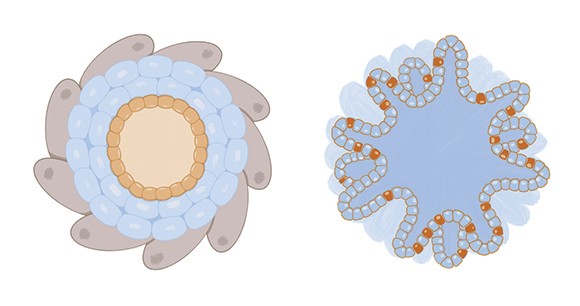
In some studies, biological effects observed via organ chips have proven to be of greater human relevance than effects observed in experiments using animals. In one example, “liver chips” were used to evaluate 27 drugs. All of the drugs were characterized as “safe” based on animal testing, but subsequent clinical trials revealed that a number of them were toxic to humans. In contrast to animal testing, liver chips correctly identified 87 percent of the toxic drugs.
In silico techniques are experiments conducted by computers via techniques such as computational modeling and artificial intelligence. AI, for instance, can screen large amounts of data from thousands of studies in a matter of moments and use that data to create infinite simulations to test how compounds might interact with each other or with human cells. Through those simulations, AI can make predictions about which compounds are toxic or which drugs could be effective in treating human diseases. In silico techniques can save time, resources, and human and animal lives by eliminating trial-and-error experimenting on animals and generating potential solutions not previously considered.
NAMs within the current research landscape
A common misconception is that recent advancements in NAMs allow for full replacement of all ongoing animal experimentation. The reality is more nuanced. As explained in an August 2024 Scientific American article, we are closer to replacing animals in regulatory product safety and toxicity testing than in “basic” research undertaken to advance general scientific knowledge.
Specifically, safety testing of cosmetics and personal and household products will likely be the first to transition away from animal use—due in large part to consumer demand for “cruelty-free” products. The United States is also making moves to reduce the use of animals for safety testing of certain drugs and pharmaceuticals: In 2022, the FDA Modernization Act 2.0 did away with a 1938 requirement to test every drug on animals before it goes to market, and in 2025, the Food and Drug Administration announced it would begin phasing out animal testing for monoclonal antibody therapies and other drugs. Toxicity testing of chemicals in consumer goods that may disperse into the environment (e.g., those found in pesticides) will likely be next to scale up NAMs, which have proven to be very effective at predicting whether a substance will cause adverse reactions in humans.
Basic research studies, on the other hand, ask broader questions about how biological systems work and often investigate behavioral or neurological outcomes that are challenging to reproduce outside living organisms. Because of the complexity and exploratory nature of this research, it will likely take much longer to replace animals with nonanimal methods in this arena than in testing. Unfortunately, many more animals are used for basic research purposes than for regulatory testing purposes.
This reality notwithstanding, in many cases where nonanimal alternatives do exist, scientists continue to rely on animals not for their scientific merit but due to other factors, such as lack of access to NAMs, lack of knowledge or training for their use, resistance to change, regulatory obstacles, and real or perceived financial costs. Many of these barriers can and must be addressed through regulatory changes and increased funding for NAM development and implementation. A huge societal and scientific shift will be required to help dismantle these barriers—but the shift is already underway.
For more information and NAM-related resources, visit awionline.org/NAMs.